The FDA has established a new safety-reporting paradigm for drugs being studied in clinical trials. A new regulation provides guidance on causality assessments for adverse events and requires aggre.. FDA Adverse Events Reporting System (FAERS) Public Dashboard. The FAERS public dashboard is a new, user-friendly and interactive web-based tool that was created to give the public the ability to query the FDA FAERS database and improve transparency. The data presented in the FAERS public dashboard has several key limitations.

Serious Adverse Event Reporting Form, Gene Transfer Protocol Policies

FDA Issues Guidance on Postmarket Adverse Event Reporting During
Reporting Adverse Drug Reactions Definitions of Terms and Criteria for

3. Overview of perinatal adverse event review pathway Maternity and

Risk Management + Adverse Event Reporting FDA Guidance AssurX

Pharmaceuticals Free FullText Adverse Events Associated with

FDA Regulation of Prescription Drugs NEJM

Safety Report Review Calls for More Thorough Adverse Event Reporting by FDA
Situation Report Template Form Fill Out and Sign Printable PDF

There’s an app for that FDA crowdsources adverse event reporting

Adverse Drug Events Occurring in U.S. Hospitals Agency for Healthcare

Adverse Events Report

Adverse Drug Reaction reporting form Openi
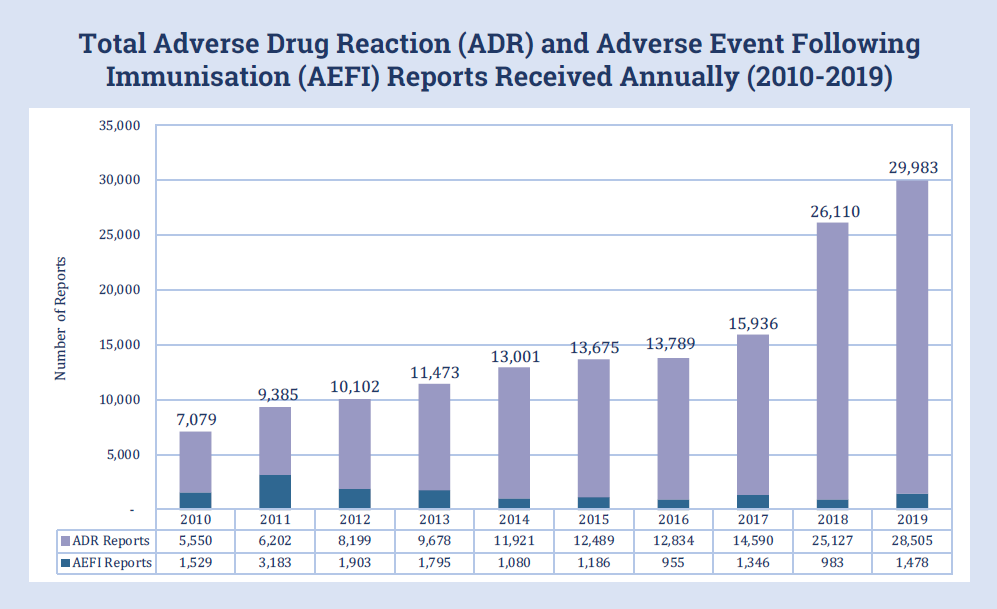
National Centre for Adverse Drug Reaction Monitoring Annual Report
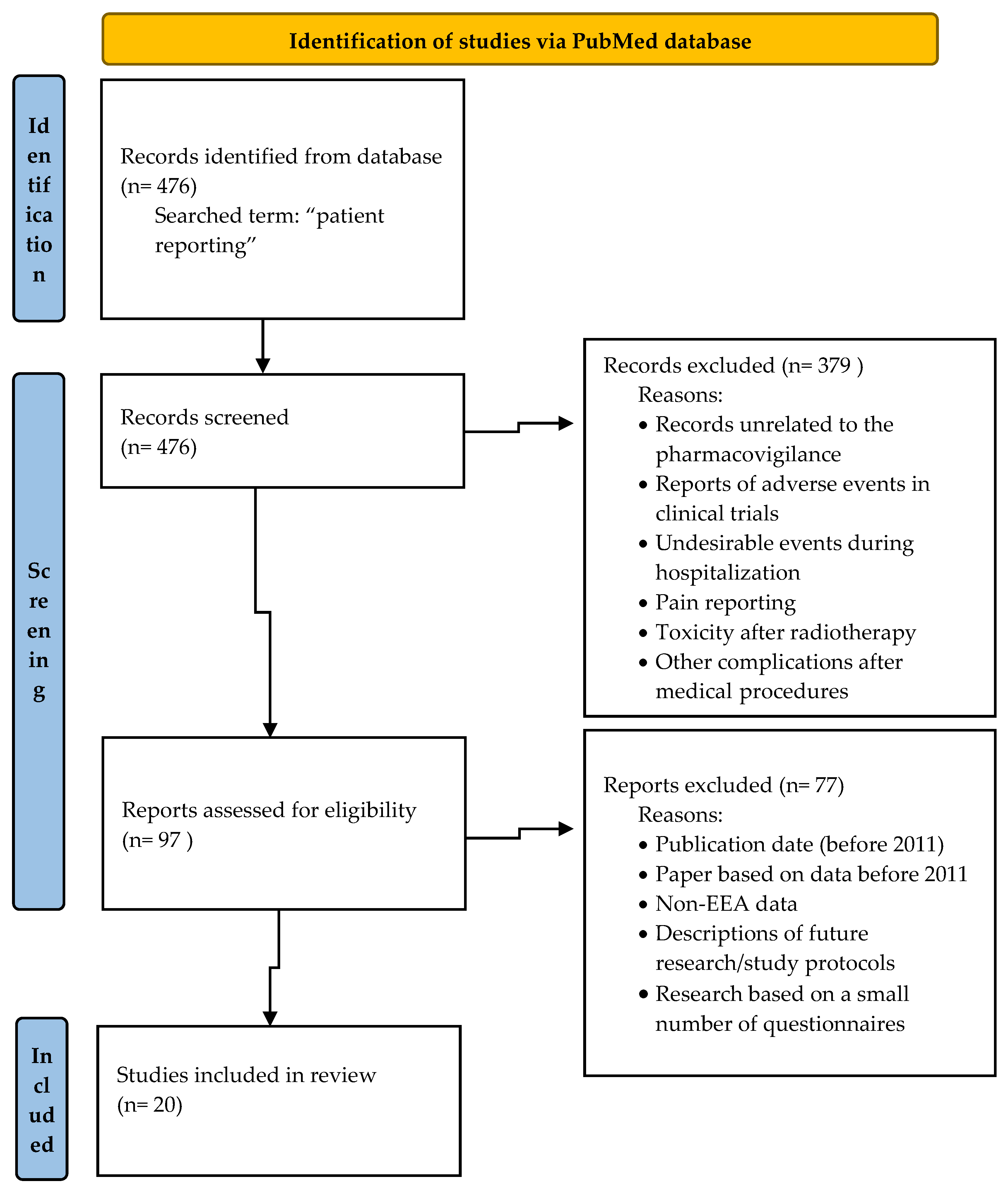
IJERPH Free FullText The Importance of Direct Patient Reporting of

Guide to Safety Reporting for Drugmakers FDA Adverse Event

Psychiatric disorders associated with immune checkpoint inhibitors a
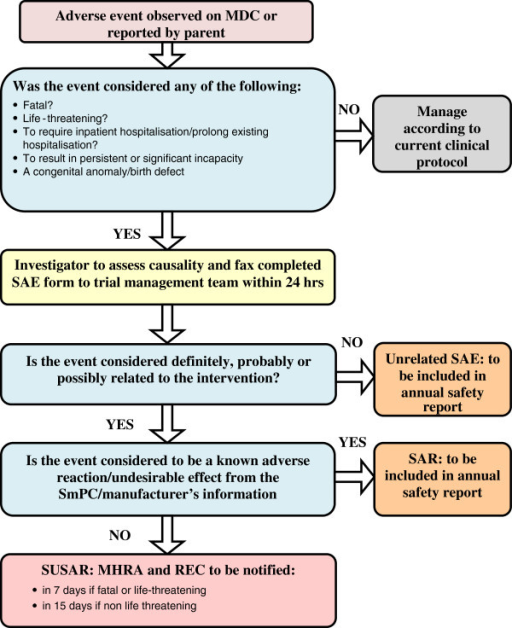
Flow chart for Adverse Event Reporting Procedures. Openi

Vaccine Adverse Event Reporting System (VAERS)

Medical Device Adverse Event Reporting Regulations EU vs. US
The FDA CFSAN Adverse Event Reporting System (CAERS) is a post-marketing surveillance system that receives and monitors adverse event and product complaint reports for foods, dietary supplements.. Meta-analyses of adverse events related to Doxil have primarily focused on side effects in ovarian cancer and breast cancer cardiotoxicity. In recurrent ovarian cancer, the toxicity profile of.